RADIO CHARACTERISTIC AND TECHNICAL SPECIFICATIONS OF IMPLANTABLE MEDICAL DEVICES IN INDONESIA WITH IMPLANTABLE ANTENNA SIMULATION AS PROOF - Dalam bentuk buku karya ilmiah
MUHAMMAD KINAN IBRAHIM

Informasi Umum
Kode
24.05.497
Klasifikasi
000 - General Works
Jenis
Karya Ilmiah - Thesis (S2) - Reference
Subjek
Regulation Of Telecommunication
Dilihat
334 kali
Informasi Lainnya
Abstraksi
In Indonesia, with a large population, the demand for biomedical devices is crucial to meet increasing healthcare needs. Currently, the use of Implantable Medical Devices (IMDs) in Indonesia is not detailedly regulated. Establishing standards for IMDs is critical due to their impact on health and well-being. IMDs, particularly wireless ones, can monitor physiological data such as glucose levels, temperature, and heartbeat, transmitting this information to external devices. This study focuses on designing an implantable antenna as proof of concept for IMD applications. Two antenna designs were developed and simulated for implantation in Small Intestine and Scalp phantoms. The designs achieved return loss values below -10 dB, indicating good performance. SAR values were 0.070 W/kg at 5.8 GHz for the Scalp Phantom, and 0.047 W/kg at 2.4 GHz and 0.031 W/kg at 5.8 GHz for the Small Intestine Phantom, all within regulatory limits. Additionally, the EIRP values of -10.246 dBW at 5.8 GHz in the Scalp Phantom and -23.35 dBW at 2.4 GHz and -29.60 dBW at 5.8 GHz in the Small Intestine Phantom demonstrated compliance with standards. The research concluded with a Policy Brief, proposing guidelines for IMD regulation in Indonesia. These recommendations aim to establish standardized specifications and lead to formal regulations, ensuring safe and effective use of IMDs in the country.<br /> <br /> Keyword : Implantable Medical Devices, Miniaturized, Metamaterial, Implantable Antenna, Biomedical
- TTI7G3 - ANTENA LANJUT
- TTH522 - DASAR DASAR REGULASI TELEKOMUNIKASI
- TTI6N3 - KEBIJAKAN DAN REGULASI TELEKOMUNIKASI DIGITAL
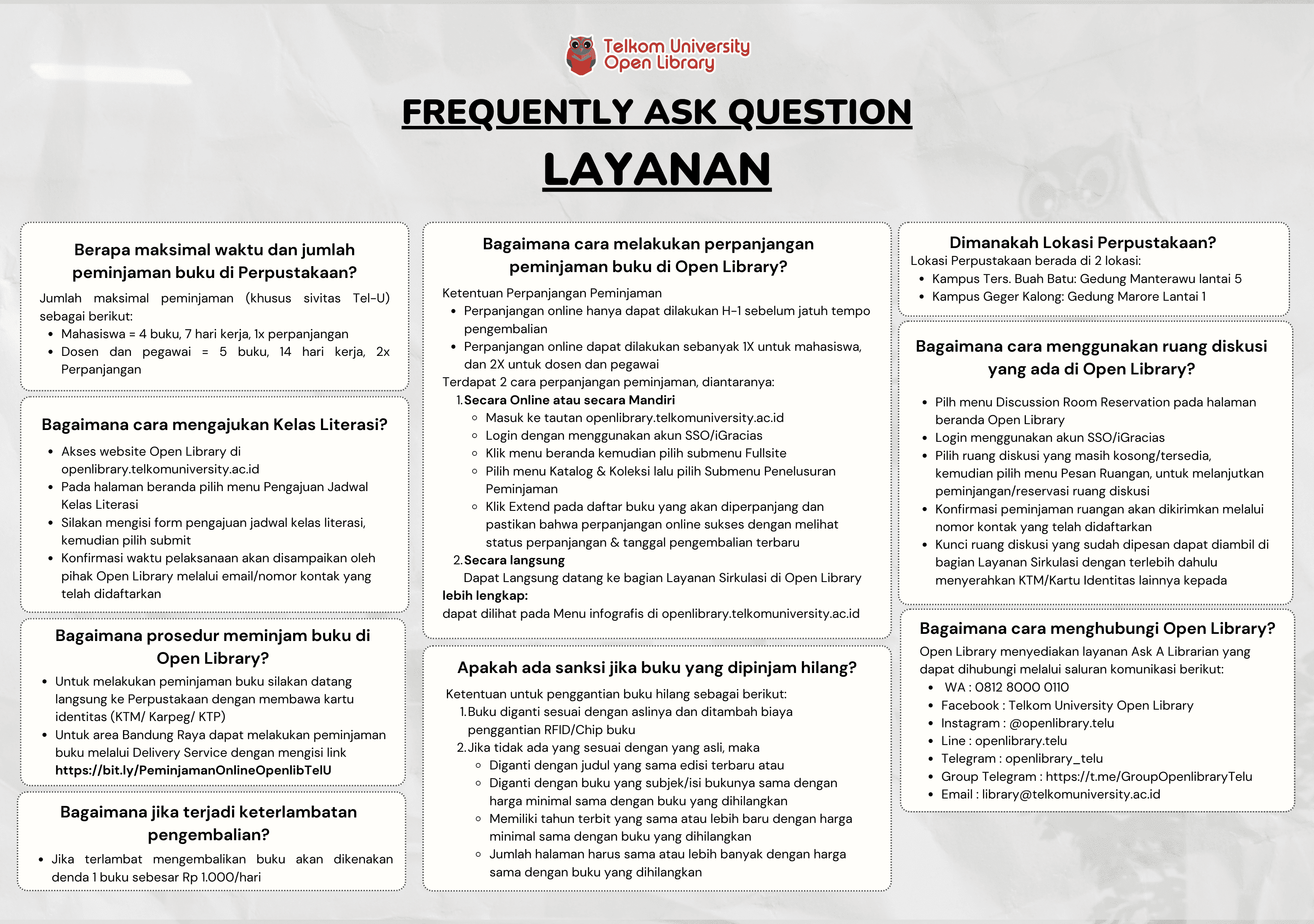
Koleksi & Sirkulasi
Tersedia 1 dari total 1 Koleksi
Anda harus log in untuk mengakses flippingbook
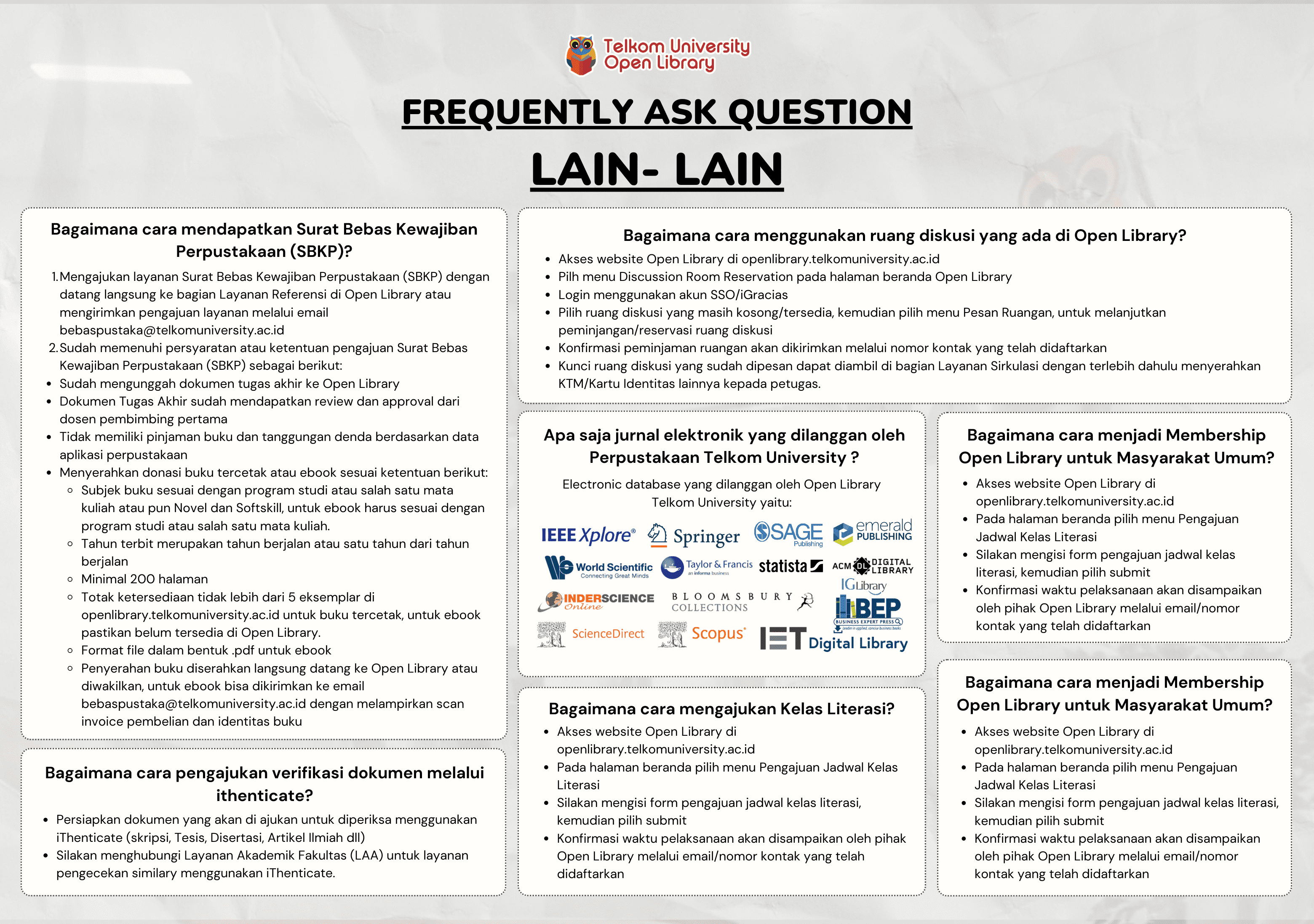
Pengarang
| Nama | MUHAMMAD KINAN IBRAHIM |
| Jenis | Perorangan |
| Penyunting | Rina Pudji Astuti, Miftadi Sudjai |
| Penerjemah |
Penerbit
| Nama | Universitas Telkom, S2 Teknik Elektro |
| Kota | Bandung |
| Tahun | 2024 |
Sirkulasi
| Harga sewa | IDR 0,00 |
| Denda harian | IDR 0,00 |
| Jenis | Non-Sirkulasi |